Leveraging Network Analysis For Drug Target Discovery
Exploring the Role of Network Analysis in Identifying Potential Drug Targets
Liked this piece? Show your support by tapping the “heart” ❤️ in the header above. It’s a small gesture that goes a long way in helping me understand what you value and in growing this newsletter. Thanks so much!
Leveraging Network Analysis For Drug Target Discovery
In biological systems, proteins, genes, and metabolites do not function in isolation. Rather, they form vast, interconnected networks that drive essential processes such as signal transduction, metabolic pathways, and gene regulation. These networks illustrate how components influence one another, shaping the dynamics and functionality of biological systems. Traditional approaches often focus on individual molecules or processes, providing detailed but fragmented insights. In contrast, network analysis offers a systems-level perspective, enabling the exploration of coordinated behaviors and identification of key players within these complex systems.
At its core, a network is a mathematical representation consisting of two primary elements: nodes and edges. Nodes represent the individual components of the system, such as proteins, genes, or metabolites, while edges denote the connections or interactions between them. These interactions can vary in nature—edges may be directed, undirected, or bidirectional, and they may even contain regulatory features, as demonstrated below:
Taken together, nodes and edges form graphs, which are the principal representations of networks. Intuitively, nodes in a network can only be connected by edges, and edges can only be connected with a node between them. For example, in a transcriptional regulatory network, a directed edge from gene A to gene B indicates that gene A regulates gene B. In contrast, protein-protein interaction networks are typically undirected, as the interactions are mutual. Similarly, metabolic pathways can be represented as graphs where nodes correspond to metabolites and edges correspond to chemical or enzymatic reactions. However, to capture the regulatory complexity of metabolic systems, more advanced representations such as bipartite graphs may be used, where distinct types of nodes (e.g., metabolites and enzymes) are included to provide a more nuanced view.
One of the key properties of a network is its structure, which can be characterized by metrics like the clustering coefficient. The clustering coefficient (Cn) quantifies how densely connected a node’s neighborhood is, ranging from 0 (no connections between neighbors) to 1 (a fully connected clique). For undirected graphs, the clustering coefficient is calculated as Cn = 2e / k(k-1), where k is the number of a node’s neighbors (i.e., the nodes it is directly connected to), and e is the number of edges shared between those neighbors. For directed graphs, the formula CN is e/k(k-1) is slightly adjusted but conceptually similar. Biological networks often exhibit high clustering coefficients compared to random graphs, suggesting that these systems are organized in a highly structured manner rather than randomly.
Another hallmark of many biological networks is their scale-free nature. In a scale-free network, most nodes have relatively few connections (low degree), while a small fraction of nodes, called hubs, have an exceptionally high degree. This distribution contrasts with random graphs, where nodes tend to have a more uniform degree distribution. Scale-free networks have several important properties. For example, they are highly robust to random node removal. Removing a random node from the network typically has little effect on overall connectivity. However, targeting a hub—a highly connected node—can disrupt the network significantly, underscoring the critical role hubs play in maintaining network integrity. This property has profound implications for drug development, as hubs often represent promising therapeutic targets.
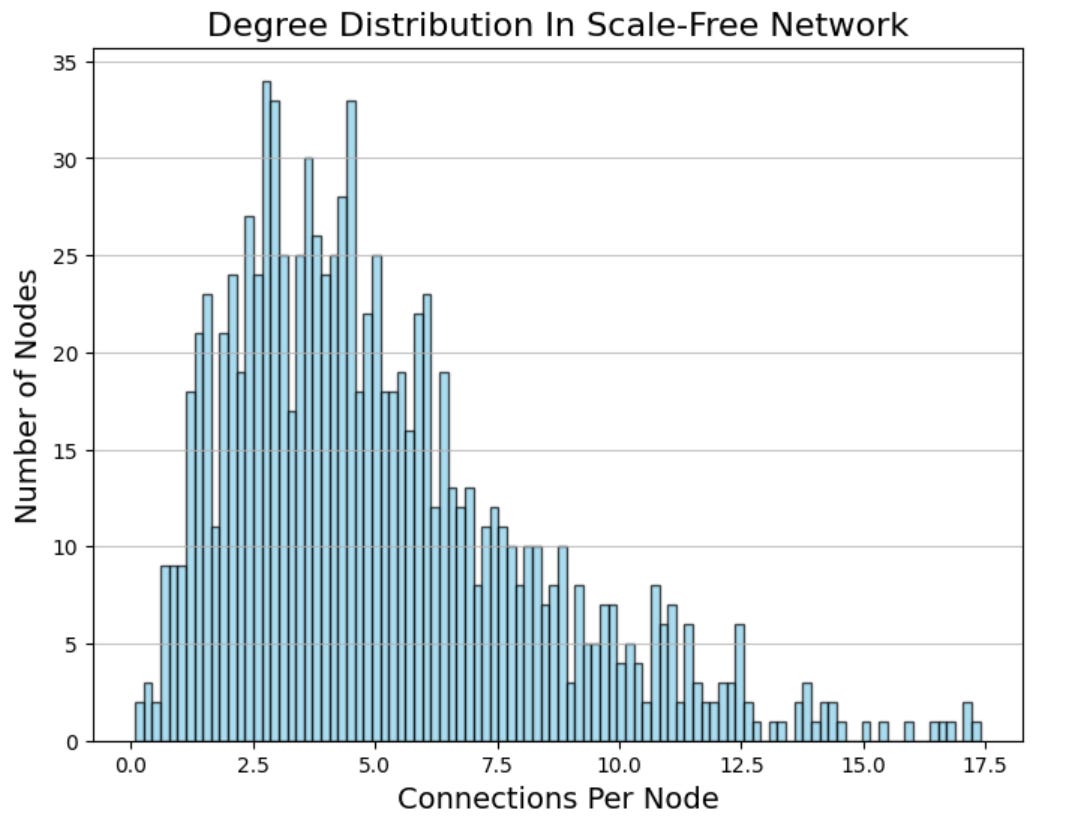
The efficiency of scale-free networks is another key feature. The average shortest path—the minimum number of steps needed to connect two nodes—in a scale-free network is significantly shorter than in random graphs of comparable size. This efficiency in information transmission is crucial in biological systems, where rapid communication between components can be essential for proper function.
In addition to the clustering coefficient, other centrality metrics can be used to identify hubs and key players in a network. Two commonly used metrics are degree centrality and betweenness centrality, which i’ve discussed at length in a previous article titled From Genes to Networks: Uncovering Biological Insights through Protein-Protein Interactions.

Degree centrality simply counts the number of edges connected to a node. Nodes with high degree centrality are considered hubs because they directly interact with many other nodes. In protein-protein interaction networks, hubs identified by degree centrality are often essential for maintaining cellular function and can make effective drug targets. Their removal tends to have a more substantial impact on network structure and functionality compared to the removal of low-degree nodes.
Betweenness centrality, on the other hand, measures how often a node lies on the shortest path between other nodes in the network. Nodes with high betweenness centrality act as bridges, facilitating communication and information flow between different parts of the network. Targeting hubs based on betweenness centrality can also be effective in disrupting network functionality. Interestingly, these targets often have fewer off-target effects and lower toxicity compared to degree-central hubs. This is because they tend to occupy strategic positions in the network rather than being involved in numerous direct interactions, which can lead to broader systemic effects when disrupted.
To illustrate these concepts, consider a protein-protein interaction network analyzed following differential expression analysis of RNA-sequencing data. Identifying hubs within this network can provide valuable insights into potential drug targets. For example, targeting a hub based on degree centrality could disrupt numerous protein interactions, effectively silencing a key node in the network and potentially halting a critical biological pathway. This approach is particularly relevant in diseases like cancer, where hyperconnected proteins may drive uncontrolled cell growth or survival. However, while targeting hubs with high degree centrality can be impactful, it may also increase the risk of off-target effects or toxicity due to the wide-ranging influence of these nodes.
On the other hand, targeting a hub with high betweenness centrality might reduce the flow of information or signaling across the network. These hubs often serve as bottlenecks or bridges between distinct network modules. For example, in signaling networks, a hub with high betweenness centrality might connect upstream signals to downstream effectors. Disrupting such a node could block pathological signaling cascades while preserving other network functions. This makes betweenness-central hubs attractive drug targets, as they often allow for more precise interventions with fewer unintended side effects.
In drug discovery, identifying and prioritizing hubs based on these centrality metrics is a powerful strategy. For instance, researchers can integrate protein-protein interaction data with differential expression analysis to pinpoint disease-relevant hubs. Computational simulations can then predict the effects of targeting specific nodes, helping to identify candidates for experimental validation. Importantly, network analysis also enables the identification of compensatory pathways or redundant interactions, which can inform combination therapies designed to overcome resistance mechanisms.
The study of biological networks not only enhances our understanding of the fundamental principles governing cellular processes but also provides practical tools for addressing challenges in medicine and biotechnology. Whether exploring how clustering coefficients highlight organizational patterns or leveraging centrality metrics to identify drug targets, network analysis opens new avenues for deciphering the complexity of life at a systems level.







